Graphene-based electronics
Graphene is derived from the same material as pencils. Graphene is formed by tightly-knit carbon atoms arranged in a sheet only one atom thick. It’s 200 times stronger than steel, yet it is flexible, stretchable, transparent, more conductive than copper, and has good heat dissipation. A single square-meter sheet of graphene can support up to 4 kilograms despite weighing just 0.0077 grams itself.
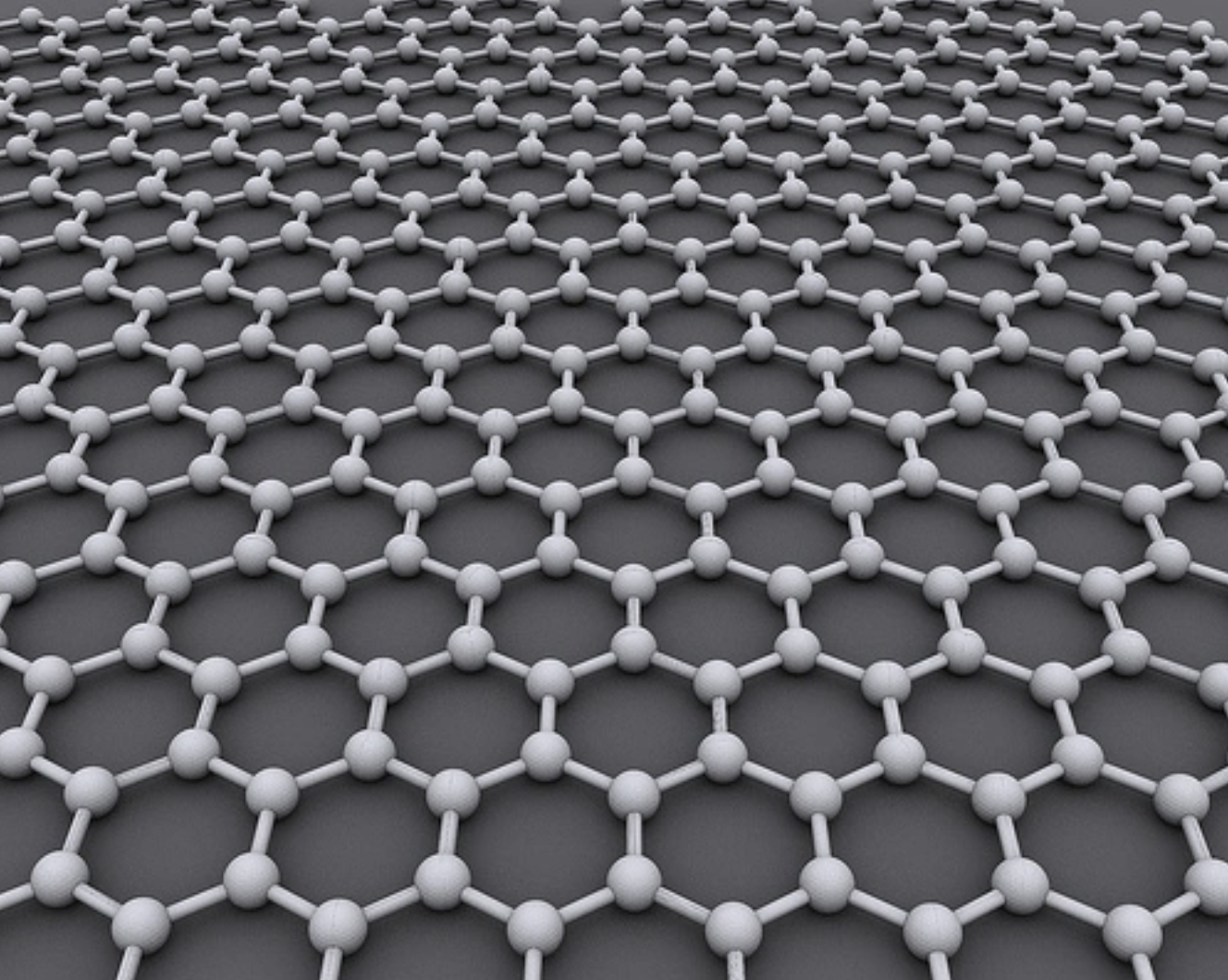
Graphene structure. Image credit: AlexanderAlUS
Graphene was originally discovered in 2004 by Andre Geim and Kostya Novoselov, professors at the University of Manchester (UK), and for this discovery, they won the Nobel Prize in Physics in 2004. It has multiple applications, including:
- Biomedical devices
- Energy
- Electronics
- Sensors
- Composites
Unfortunately, graphene doesn’t have a bandgap like silicon and this is currently limiting its use as a silicon replacement in integrated circuits. While the 2D carbon material has no bandgap, bilayer graphene can be tuned to have a bandgap. Bilayer graphene films on silicon carbide can be better controlled. Trilayer graphene can also be tunable to produce a bandgap to develop field-effect transistors in a semiconductor device.
You can read more about the enablers that lead to this on our recent blog post Graphene. The miracle material and these past ones Graphene and Carbon Nanotubes Part 1 and Part 2.